Biorez has pioneered the BioBrace, the first ‘biocomposite’ soft tissue scaffold engineered to both mechanically reinforce tendon and ligament repairs, and enhance healing through its unique material properties and open architecture. The BioBrace is recently FDA approved for the reinforcement of soft tissue repairs such as anterior cruciate ligament (ACL) in the knee, rotator cuff in the shoulder, and Achilles tendon in the ankle.
Biorez designed the BioBrace to potentially increase the durability of currently available tendon and ligament repairs and shorten the healing time. For example, prolonged recovery following ACL reconstructive surgery remains a challenge for both the patient and surgeon.
The basis of the BioBrace is a highly porous collagen scaffold, with pore openings of various sizes within a biological range, augmented with biocompatible microfilaments that structurally reinforce the scaffold. Its biomechanical profile enables load sharing to support ligament and tendon repair throughout the healing process.
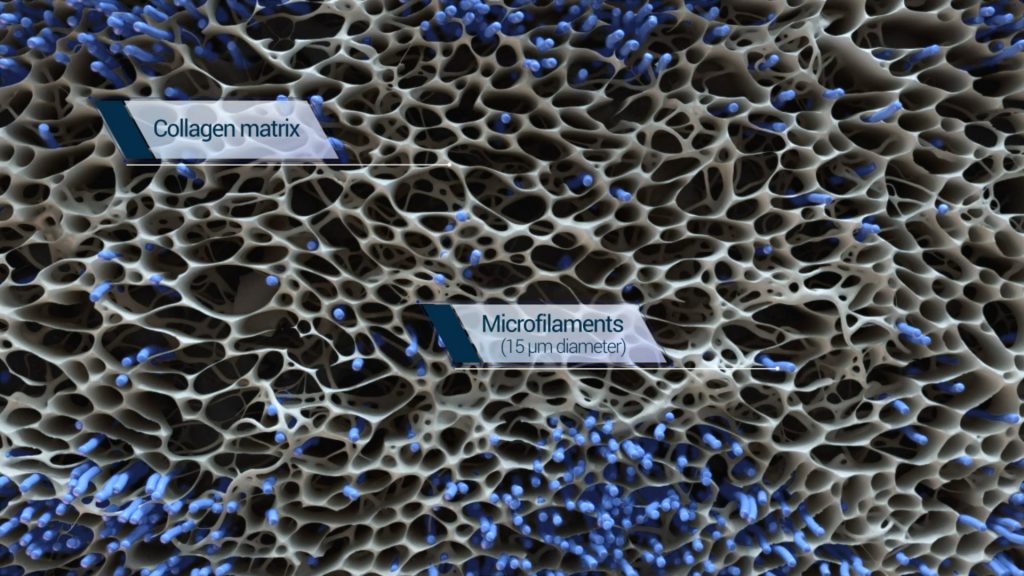
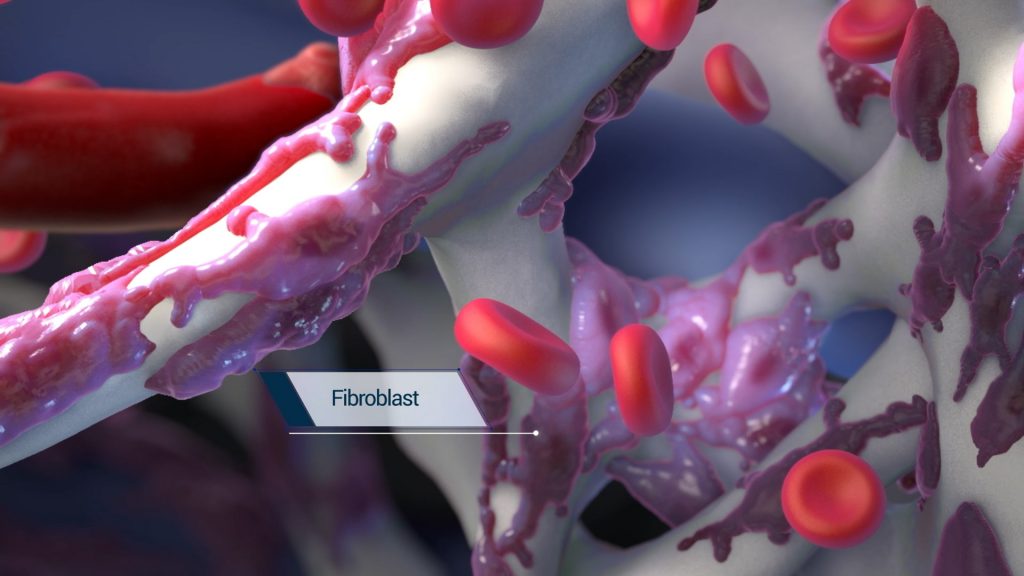
The engineered structure of the BioBrace is also designed to facilitate regeneration and ingrowth of blood vessels and fibroblasts into the scaffold, thereby integrating with the native surrounding tissue.
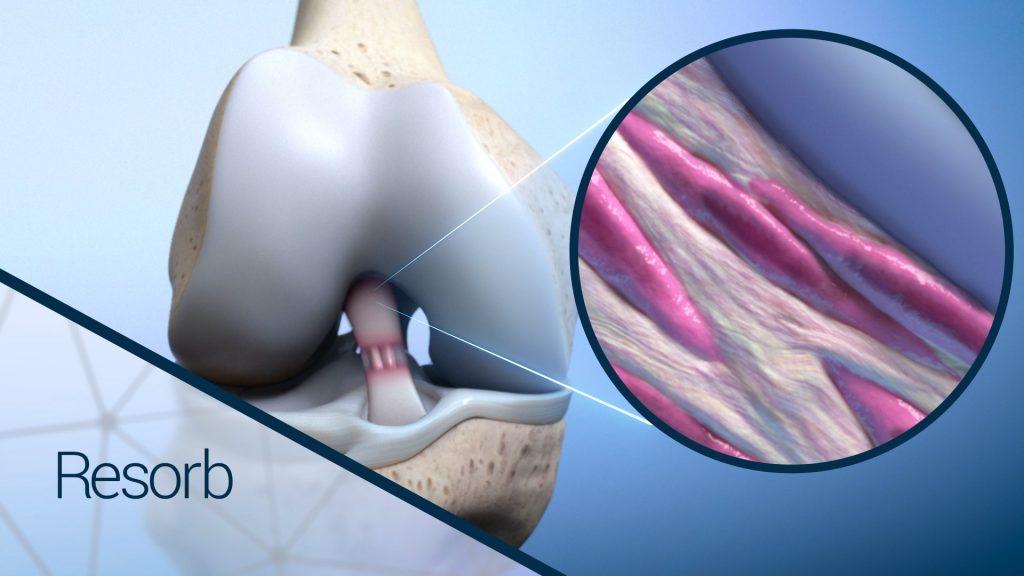
As the healing process continues after graft implantation, there is a gradual increase in mechanical loading to the newly generated tissue that coincides with the gradual resorption of the BioBrace scaffold, until the anatomy is restored to its normal state.
Biorez CEO Kevin Rocco reached out to Mike Astrachan at XVIVO for their medical animation needs after initially meeting at events held by BioCT, the Connecticut organization for the bioscience community. XVIVO is always excited to work with other Connecticut-based bioscience companies and to be a part of advancing the regional industry toward better solutions for patients and healthcare challenges.